







Because our brains
and mental health
deserve precision
medicine for the
greater good
The Women’s Brain Foundation (WBF) is an international non-profit organization based in Switzerland harnessing the brilliance of a global team of scientists. We are experts from various disciplines who work with patients and caregivers towards the implementation of sex and gender within precision medicine, from basic science to novel technologies.
"From patients'
differences to patients'
characteristics, to achieve precision medicine and render healthcare systems sustainable."
Dr. Antonella Santuccione Chadha

"From patient's
differences to patients'
characteristics, to achieve precision medicine and render healthcare systems sustainable."
Dr. Antonella Santuccione Chadha
We bring Swiss precision to medical treatment and care to ensure sustainability in healthcare.
Men and women are different when it comes to disease risks – frequency, severity, symptomatology, diagnostic journey and even response to treatments. Our mission is to transform the development of drugs and medical treatments through sex and gender factors as a gateway to precision medicine and care. We do this by running research projects, consult with industry partners, engage with regulators, government and non-governmental organisations and finally, develop novel technologies to bring new treatment options to patients & care-givers.
Through these four pillars, we are making the change a reality:
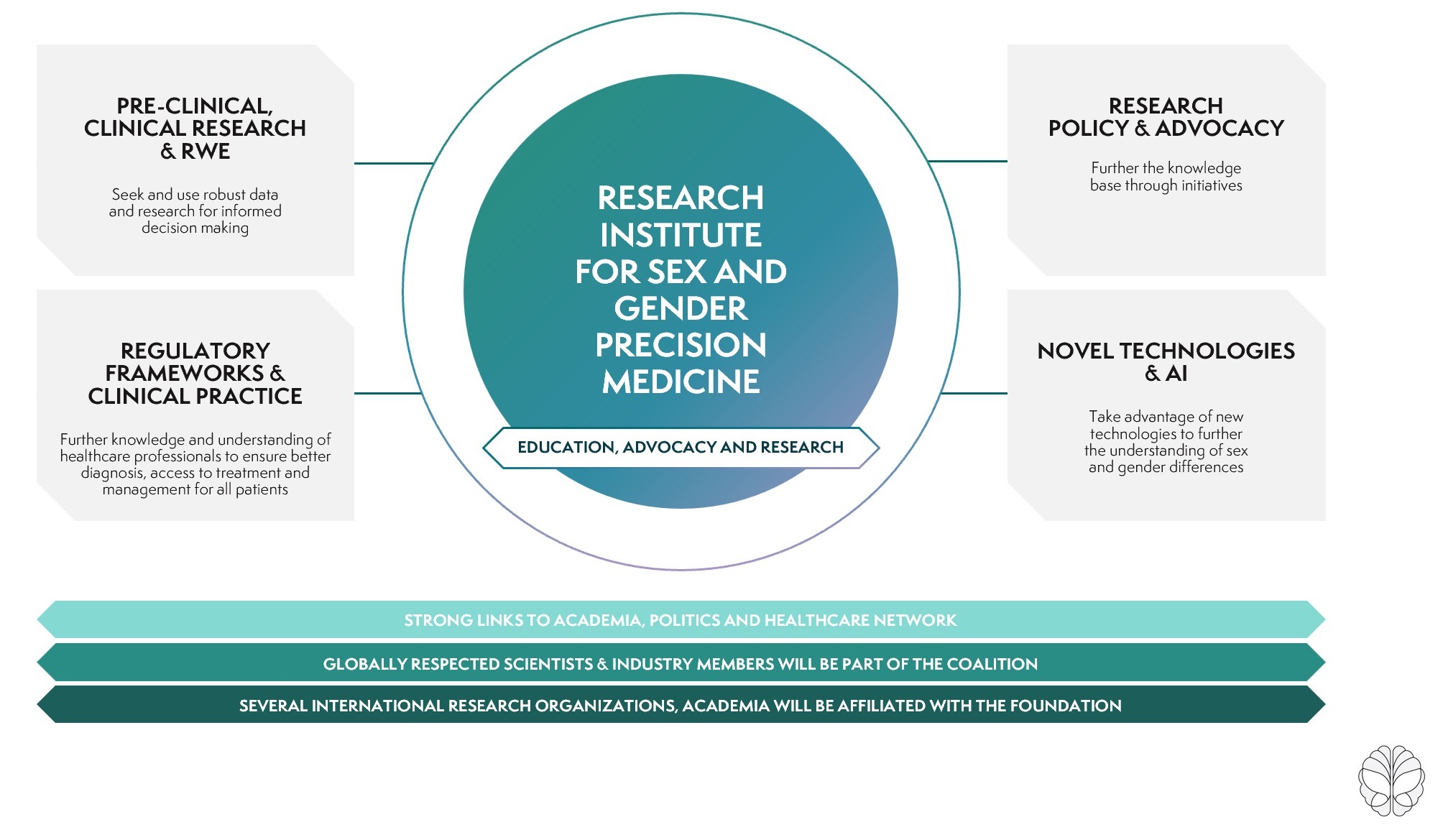
Our contribution for more clarity.
One of the main goals of the Women’s Brain Foundation is to generate new evidence on sex and gender differences in brain and mental diseases. See the Impact we are having through our publications.
Where knowledge becomes an eventful journey.
We love to share our expertise with all who want to change the future by organizing a variety of events and workshops.
International Forum on Women’s Brain
and Mental Health
International Forum on Women’s Brain and Mental Health
The Women’s Brain Foundation International Forum on Women’s Brain and Mental Health is the organization’s flagship event.
Workshops and webinars
Many of the world’s largest organisations help us fund our research by partnering with us to deliver talks on topics such as #MentalHealth & self-care at work. Let’s do the same for your organization—
Our formats are flexible and focused, ranging from simple discussions to half and full-day sessions. Contact us to setup a time to discuss.
Always up to date.
This was one of the feedback received from an attendee at last Friday’s lunchtime MedTalk organized by the @Health Science Club –...
Read MoreWe are thrilled to share our recent experience presenting at the OneNeurology meeting, where we showcased the impactful work of...
Read MoreWe are delighted to announce our latest opinion article in Frontiers in Global Women’s Health titled, “The Impact of Informant-Related...
Read MoreGet to know
Sofia Petersson
Sofia Petersson from Sweden was diagnosed with Alzheimer’s Disease in her late 30s. Her father, uncle and grandmother also suffered from the disease. Sofia contributed a blog for the Women’s Brain Foundation about living with Alzheimer’s.
Our expertise and scientific advice.
Based on our unique medical, scientific and regulatory expertise, WBF can advise on research pipelines, policy and practice in corporate and academic settings. For more information, please contact us.










